
Getting a drug approved in India sounds simple on paper. In reality, it’s anything but. Many companies spend millions, follow the science, and still end up stuck in delays or outright rejection. Not because the drug is bad, but because the process is strict, detailed, and easy to mess up.
India’s drug approval system leaves very little room for error. Small mistakes in classification, dossiers, or compliance can cost months, sometimes years. That’s why understanding the actual drug approval process in India matters more than just knowing the rules exist.
This guide breaks the process down step by step. No theory. No fluff. Just a clear look at how approvals work, where companies usually fail, and what you must do even after your drug gets approved. The goal is simple: help you avoid expensive mistakes and move faster, with fewer surprises.
New Drug Approval Process in India at a Glance
So the new drug approval process in India has around 8 steps:
- Drug identification
- CTD Dossier prep
- Application submission
- Clinical trials
- CDSCO Technical evaluation
- Site Inspection
- Marketing/Import License grant
- Post marketing surveillance
Now among these steps Dossier preparation is the most important step, and many companies fail to meet the expectations here only. Most of the important step of the complete drug approval process in India is preparation on the dossiers, we will delve deeper into in the coming sections. First let’s jump to main step i.e. drug classification.
Drug Classification
So let us start with the number one step which is to identify your drug, there are basically three main categories into which you can classify your drug which are:
| New Drug | A molecule that has never entered India |
| Fixed-dose Combination | A new combination of two or more drugs in a fixed ratio |
| Investigational New Drug | A molecule that is still under research or trials and is not yet approved to market yet. |
You may get a benefit of skipping the clinical trials if your drugs are already registered in US, Europe or Japan. Under rule 122A of drugs and cosmetics act, drugs that are already registered in these countries may not have to undergo trials in India.
But the final decision still depends on CDSCO. Now let’s start with the most important step, which is preparing the Common technical document. And this is the most important part of your drug application, as this is the place where major number of companies mess up.
Preparing the Common Technical Document
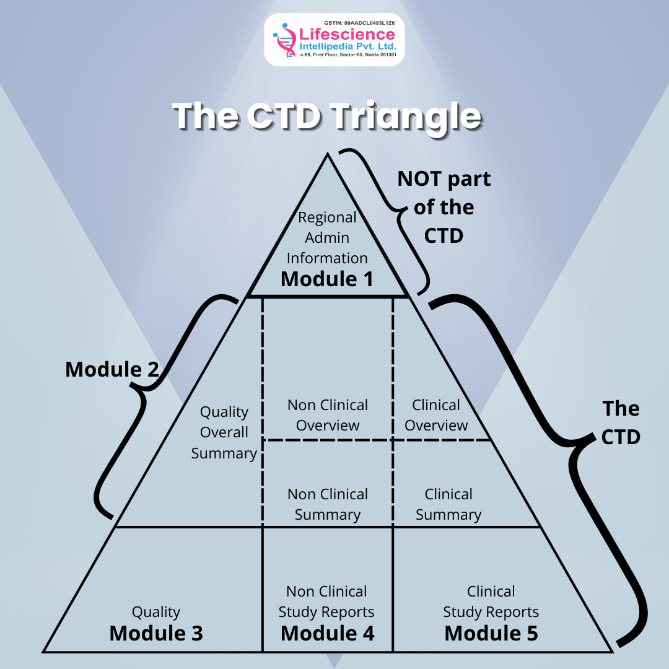
Common technical document or commonly called CTD, is the soul of your complete drug approval in India. In very simple terms, you can understand it as a complete summary of your product. It contains a total number of 5 modules and each modules contains important information about your drug.
CTD are reviewed by CDSCO, and if your CTD is not up to mark, your approval will definitely get delayed or even rejected. A lot of our clients had the same issue, their product was up to the mark, but because they didn’t put efforts on their CTD, their application delayed to months or even up to a year.
Here are the 5 modules that your CTD must include:
| Modules | Description |
| Module 1 | First module of any CTD contains regional administrative information. In simple terms if you are applying to India, it should have the documents that are critical for Indian regulatory bodies such as Form 44, Treasury challan fee or the proposed label for use in India. |
| Module 2 | The second module contains that summary of the third, fourth and fifth modules. Summaries such as general introduction to the drug, pharmacological class, mode of action, and proposed clinical use. In addition to that, including the quality, clinical and non-clinical summaries must also be present in this module |
| Module 3 | Third section is all about the quality of the drug, it contains information on Chemistry, manufacturing and control. One pro tip here- make specific sections for drug substance and drug products. Also, your manufacturing process, batch consistency, analytical methods and stability data is mentioned here. |
| Module 4 | In the 4th module you need to add the non-clinical study reports and toxicological studies. The overview, needs to have pharmacologic, pharmacokinetic and toxicologic evaluation of the drug. |
| Module 5 | Fifth module describes the efficacy and clinical reports of your drug. |
Expert tips to create a good CTD
- Ensure that your CTD is exactly in the above mentioned structure. This structure ensures that it is acceptable for drug approvals in India.
- The next tip is storytelling. In the 2ndand 3rd module you can take some creative liberty to present your drug a way that the reviewer can see the bigger picture, and not just the molecule you have applied to get approval of.
- Last is, to present all the data in simple terms without making any false or too good to be true claims, as this might lead to rejection of your application.
Most drug approvals in India don’t fail because of the product. They fail because of a weak CTD. Lifescience Intellipedia helps pharma companies build CDSCO-ready CTDs that are clear, complete, and reviewer-friendly. From module-wise gap analysis and data structuring to compliance checks aligned with Indian regulations, we help reduce queries, delays, and rework.
If your CTD is in progress, or already stuck in review, Lifescience Intellipedia can help you fix it before it costs you months.
You might also like: “Common Pharmaceutical Market Entry Barriers and How to Overcome Them”
Application, CTD Evaluation and Clinical Trials

Courtesy: CDSCO
Now that you have prepared the CTD, now is the time to apply, you can apply through SUGAM portal, you need to fill the Form 44 and submit the fees and get your reference number.
CDSCO Technical Evaluation
After application, CDSCO will start evaluating your dossiers. Here are the key things they evaluate in your CTD:
- Clinical data
- Safety margins
- Pharamcokinetics.
- GMP compliance verification
Your application is under the review by the Subject Expert Committee or SEC.
Now here’s why it is importatn to focus more on the CTD, if the data in CTD is not clear, simple and elaborative, additional data, clarifications can be asked, and this is another reason why drug approvals are often delayed.
Clinical Trials
Now after the CTD is approved, you need to conduct the clinical trials for your drug, Follow schedule Y of the Drugs and Cosmetics Act. The trials protocols needs to be approved by an Ethics Committee before even starting with the trial. And this leads to a series of paperwork, and restarting with the work again. Here is how clinical trials are conducted:
| Phase | Purpose | Participants |
| Phase I | Safety and dosage | Healthy volunteers |
| Phase II | Side effects of drug and efficacy | Small patient groups |
| Phase III | Confirmatory data | Large patient population |
| Phase IV | Post-marketing surveillance | Real-world settings |
If your drugs are already approved in US, Europe or Japan, you can avail a partial or full waiver you just need to submit the clinical trial reports from there,
Site Inspection
Alright now, is the time for site inspection. Indian government is very strict when it comes to approving a new drug, therefore, they may require a site inspection. During site inspections, they often check for-
- GMP Compliance
- Data Integrity
- Batch consistency
- Equipment validation
- ethical trial conduct, (in case of clinical sites)
But if you have GMP certificate from US, Europe, or UK, you might pass this step. But, India still reserves the right to conduct fresh inspections, and especially for products from low-cost regions.
Market/import license grant
Now once all the formailities are done, and you meet all the CDSCO requirements, you will gain the license.
- Form 46 for manufacturing in India
- Form 45 is the import license for foreign-made drugs.
So your product is now approved, you have got the licence but as I have told you, there are still a few things which you do not do correctly, you might get your drug license rejected. And there is Post-marketing Surveillance.
Post-market Surveillance
This is the last and most important thing, after the approval you need to-
- Monitor the real-world adverse events
- File periodic safety updates
- Maintain pharmacovigilance systems
- Report any recalls, product variations or safety warnings.
Conclusion
The drug approval process in India is not impossible—but it is unforgiving. From drug classification to CTD preparation, clinical trials, inspections, and post-market surveillance, every step needs attention. Most delays don’t happen because the science is weak. They happen because dossiers are unclear, data is incomplete, or post-approval obligations are ignored.
If there’s one takeaway, it’s this: CTD preparation and post-approval compliance matter as much as the drug itself. Get these wrong, and even an approved product can face suspension.
Companies that plan early, prepare clean dossiers, and stay compliant after approval move faster and spend less fixing mistakes. In India’s regulatory system, doing things right the first time is not optional, it’s the only way forward.







