Bringing a medicine to the European market isn’t just a regulatory exercise—it’s an endurance game. A long one. The kind where preparation matters more than speed, and small mistakes quietly add months to your timeline. The Centralized Procedure (CP) is the big, shiny route everyone wants because one approval opens the doors to all EU Member States. But like most things that look clean on the outside, the real work happens behind the curtain: data gaps, dossier structure, CHMP questions, clock stops, and a whole lot of waiting.
If you’re a company planning a European launch, here’s the truth: you can either learn the CP timeline the hard way, through delays, confusion, and late-night fixes—or you can understand the system upfront and move through it with intention and clarity. Every phase has its own tempo. Every step has a bottleneck waiting for someone unprepared. And every decision you make early on compounds, positively or negatively down the line.
Let’s start at the top, with the different routes a product can take to enter the European market.
Pathways to Market Authorization in Europe
Europe gives companies a few different ways to get a medicine approved. Think of them like different doors that lead to the same house: market authorization. Some doors are big and shiny. Some ae small and boring. But each one works depending on what your product is and where you want it approved.
Here are the main pathways:
Centralized Procedure (CP)
This is the “one-shot” door. You apply once, and if things go well, you receive approval for all EU Member States at the same time.
Used mostly for:
- Big innovation drugs
- Biotech products
- Advanced therapies
Things you must know:
One application → One review → EU-wide approval. Easy to understand, hard to execute.
Decentralized Procedure (DCP)
This is for when your product is not yet approved anywhere in the EU, but you want it in multiple countries at once.
How it works:
- You pick one country as RMS (Reference Member State).
- Other countries are CMS (Concerned Member States).
- They review together like a study group, but with rules.
Good when you want broad access but don’t qualify for CP.
Mutual Recognition Procedure (MRP)
This is used when your product is already approved in one EU country, and you want others to “copy-paste” that approval.
Simple logic:
- One country says “yes”
- You ask the other countries to “also say yes”
Works for already-approved medicines expanding into more EU markets.
National Procedure (NP)
The most basic pathway. You apply to only one country, and that country alone reviews your file.
Useful for:
- Products meant for a single market
- Early launches before regional expansion
Centralized Procedure Timeline for MAA in Europe
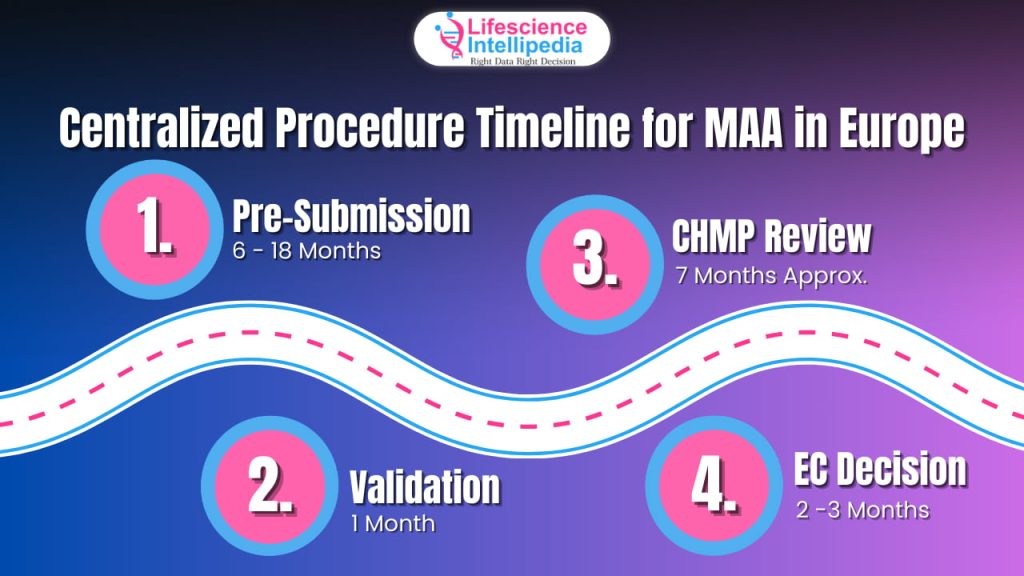
The Centralized Procedure (CP) may look like a single pathway, but the timeline behind it is long, layered, and honestly, a bit tiring. Still, it’s the fastest way to get EU-wide approval in one go. Below is the simple version of how long each stage usually takes.
Pre-Submission Activities (6–18 months)
Before your submission to MAA, you spend months preparing, checking, fixing, and double-checking every part of your dossier.
Typical activities include:
- Pre-submission meetings with EMA
- Finalizing Module 1 requirements
- Making sure quality, nonclinical, and clinical data are not a mess
- Choosing your Rapporteur & Co-Rapporteur
- Getting your product name cleared
This phase feels long because it is long — but skipping it means suffering later.
Validation (1 month)
EMA checks if your dossier is complete enough to even start the review. This is basically the “Are all your papers in the right folder?” step. If everything is good the review clock will start.
CHMP Review (210 Active Days / ~7 months)
The CHMP goes through every scientific, quality, safety, and efficacy detail.
The timeline includes:
- Day 80 List of Questions (LoQ)
- Clock Stop: You answer these questions. Your timeline now depends on how fast you respond.
- Day 120 & Day 180 Assessments
- Day 210 Final Opinion
The “7 months” is active review time. Clock stops can push the total to 12–18 months easily.
European Commission (EC) Decision (2–3 months)
After CHMP gives a positive opinion, it goes to the European Commission for the final signature.
This step includes:
- Legal checks
- Translation into all EU languages
- Final adoption
Once EC signs → Your product has EU-wide marketing authorization.
Total Timeline: 12–18 Months
From submission to approval, most companies fall in the 12–18 month window. The biggest delay factor is the clock-stop responses — if they take long, everything else stretches.
That’s why many companies work with Regulatory Affairs consulting teams such as Lifescience Intellipedia, because they help clean up gaps early and keep the whole process moving without unnecessary drama.
In the next section, we will dive deeper into the various steps involved in the Centralized Procedure.
Read article: Common Pharmaceutical Market Entry Barriers and How to Overcome Them
Steps in Centralized Procedure for Market Authorization Application in EMA
The Centralized Procedure may look complicated, but it actually follows a simple, step-by-step path. Think of it like a long checklist you need to finish before the EU says “yes” to your medicine. Here are the main phases explained in a quick, simple, and very dumb-friendly way.
1. Presubmission Phase
This is the “get your act together” phase. You prepare everything the EMA will need so your application doesn’t fall apart later.
Typical activities:
- Meet with EMA for scientific advice
- Confirm eligibility for Centralized Procedure
- Fix data gaps early
- Finalize the dossier structure
- Pick Rapporteur and Co-Rapporteur
- Schedule pre-submission meetings
If this phase is messy, the rest becomes painful.
2. Submission Phase
Here, you officially send your Marketing Authorization Application (MAA).
What happens:
- EMA receives your dossier
- They check if it’s complete (validation)
- If yes → Review clock starts
- If no → You fix issues before the clock starts
It’s like handing in your homework. If something is missing, they give it back.
3. Assessment Phase
This is the longest and most important step.
Key stages:
- CHMP starts scientific review
- You receive the Day 80 List of Questions (LoQ)
- Clock Stop begins while you respond
- Day 120 and Day 180 reviews follow
- Rapporteurs assess safety, efficacy, and quality
- CHMP prepares final opinion
This phase decides everything. Strong data = smooth ride. Weak data = delays.
4. Decision-Making Phase
Once CHMP gives a positive opinion, the application goes to the European Commission.
The EC:
- Reviews the CHMP opinion
- Processes translations
- Issues the final marketing authorization
This step is straightforward and usually takes 2–3 months.
5. Post-Authorization Phase
Approval is not the end. Now you must keep the product compliant.
This includes:
- Managing variations
- Submitting PSURs / safety updates
- Maintaining quality systems
- Handling renewals
- Meeting post-marketing commitments
Basically, you keep proving your product stays safe and effective.
How Regulatory Affairs Consulting Speeds Up Your CP Timeline?
The Centralized Procedure can easily stretch beyond 18 months when submissions are messy, responses are slow, or data gaps show up at the wrong time.
A Regulatory Affairs consulting company like Lifescience Intellipedia helps keep the entire process clean, organized, and moving. Here’s how they shorten delays through practical, hands-on support:
- Early Gap Analysis: We check your data package early to spot missing studies or weak areas before EMA does, preventing surprise questions later.
- Right Dossier Structure From Day One: We will prepare your dossier in the exact format EMA expects, reducing validation issues and making the review smoother.
- Faster Responses During Clock Stop: Our experts will help you prepare clear, accurate, and quick responses to CHMP queries so the review clock restarts sooner.
- Smooth EMA Coordination: We will handle communication with EMA, Rapporteurs, and stakeholders so nothing gets delayed due to miscommunication.
- Pre-empting National Requirements: Our team of regulatory affairs experts will plan ahead for country-specific needs (like translations and PI updates) to avoid last-minute bottlenecks.
- Compliance Checks That Avoid Rework: We conduct quality and regulatory checks to make sure the submission is right the first time, no resubmissions, no fixes later.
With the right consulting support, companies avoid the common mistakes that stretch CP timelines. We will help you focus on science while we will handle the regulatory heavy lifting.







